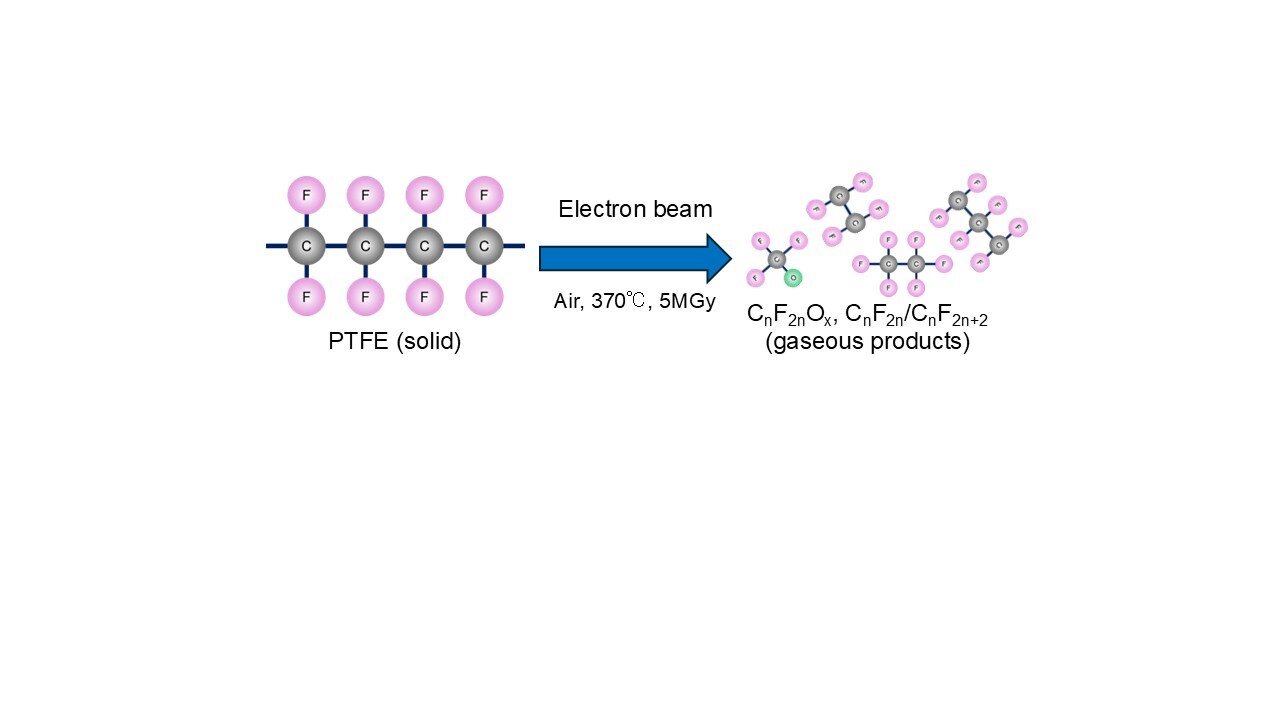
Plastics like Teflon are famously durable—and infamously difficult to recycle. But a breakthrough from researchers at the National Institutes for Quantum Science and Technology (QST) may offer a powerful new solution.
The team, led by Senior Principal Researcher Dr. Akira Idesaki, has developed a technique using electron beam (EB) irradiation to break down polytetrafluoroethylene (PTFE) into gaseous products, effectively transforming a solid, heat-resistant fluoropolymer into useful chemical components. Their results are published in the journal Radiation Physics and Chemistry.
“By applying heat during irradiation, we were able to reduce the energy required to decompose PTFE by 50% compared to traditional methods,” said Dr. Idesaki. “This makes large-scale recycling of fluoropolymers much more viable.”
PTFE—best known by the trade name Teflon—is widely used in electronics, medical devices, and nonstick cookware. Its resistance to heat and chemicals comes from its strong carbon-fluorine bonds, which also make it a member of the environmentally persistent family of substances known as PFAS, which are informally called “forever chemicals.”
Traditional methods for decomposing PTFE, such as pyrolysis, require extremely high temperatures (600-1,000 °C) and massive energy input. The QST team demonstrated that heating PTFE to 370 °C and irradiating it with an EB in the air allowed them to convert 100% of the plastic into gas.
Gases from solids: The chemistry of breakdown
The key to the method is combining heat with radiation. When PTFE powder was irradiated with a dose of 5 MGy at 30 °C, only 10% decomposed. But at 270 °C, that number rose to 86%. At 370 °C, full decomposition was achieved.
The main gases released during the process were oxidized fluorocarbons—chemical compounds that contain both fluorine and oxygen, and perfluoroalkanes—compounds containing fluorine and carbon. The research team identified them using gas chromatography and mass spectrometry. These gases could be collected and reused as raw materials in chemical industries, helping to support a more sustainable, circular use of resources.
Structural transformation and crystallinity
The researchers also found that heating PTFE during EB irradiation caused changes in its internal structure. The small crystal units inside the material became larger, which suggests that the material underwent a reorganization. Infrared and X-ray analysis showed that most of the oxidized chemicals were removed, meaning the PTFE was efficiently broken down into gas.
“High-temperature irradiation not only enhances decomposition but also changes the internal structure of PTFE,” noted the first-author Dr. Hao Yu, a researcher at QST. “This helps explain why the process becomes more energy-efficient as the temperature rises.”
Toward industrial application
Based on their data, the researchers estimate that this new approach could cut the energy cost of PTFE recycling from approximately 2.8–4 MWh per ton—typical for pyrolysis (high temperature)—by 50% using EB irradiation. This level of efficiency could make it commercially attractive for industries that generate PTFE waste.
“We hope this technology will contribute to the safer, cleaner, and more cost-effective recycling of high-performance plastics,” said co-author Dr. Yasunari Maekawa, who led the research project.
More information:
Hao Yu et al, Effects of temperature on the decomposition of PTFE induced by electron beam irradiation, Radiation Physics and Chemistry (2025). DOI: 10.1016/j.radphyschem.2025.113029
Citation:
Electron beam irradiation decomposes Teflon-like fluoroplastics efficiently (2025, July 24)
retrieved 25 July 2025
from https://phys.org/news/2025-07-electron-irradiation-decomposes-teflon-fluoroplastics.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.