An international research team led by the University of Vienna has succeeded in developing a new method to directly measure partial charges in molecules. The results, now published in Nature, provide new insights into molecular interactions and offer potential applications in drug development and materials science.
Electrostatic forces—the attractive or repulsive interactions between atoms or molecules—lie at the heart of all molecular interactions. They are fundamental to how molecules assemble, align, and respond to one another. In chemistry, these forces are described in terms of partial charges: tiny imbalances in how electrons are distributed within a molecule. These subtle shifts in charge govern how molecules interact with each other and their surroundings. They are key to understanding chemical reactivity, biological function, and material behavior.
In medicine, for instance, partial charges influence how drugs are absorbed, distributed, and metabolized—and can shape both their therapeutic effects and potential side effects. Yet despite their significance, partial charges have remained purely theoretical: until now, there was no way to measure them directly.
Breakthrough in measuring molecular charges
A research team led by Tim Grüne, Head of the Core Facility for Crystal Structure Analysis, and Christian Schröder from the Department of Computational Biological Chemistry at the University of Vienna, has now developed a method that allows partial charges to be determined experimentally.
“We used a technique called electron diffraction,” explains Grüne. “It involves directing a fine beam of electrons at a tiny crystal. Because electrons are charged, they are sensitive to the electrostatic potential within the crystal and thus to the partial charges of atoms. The resulting tiny deflections in the beam were recorded using a new camera developed at the Paul Scherrer Institute in Switzerland.”
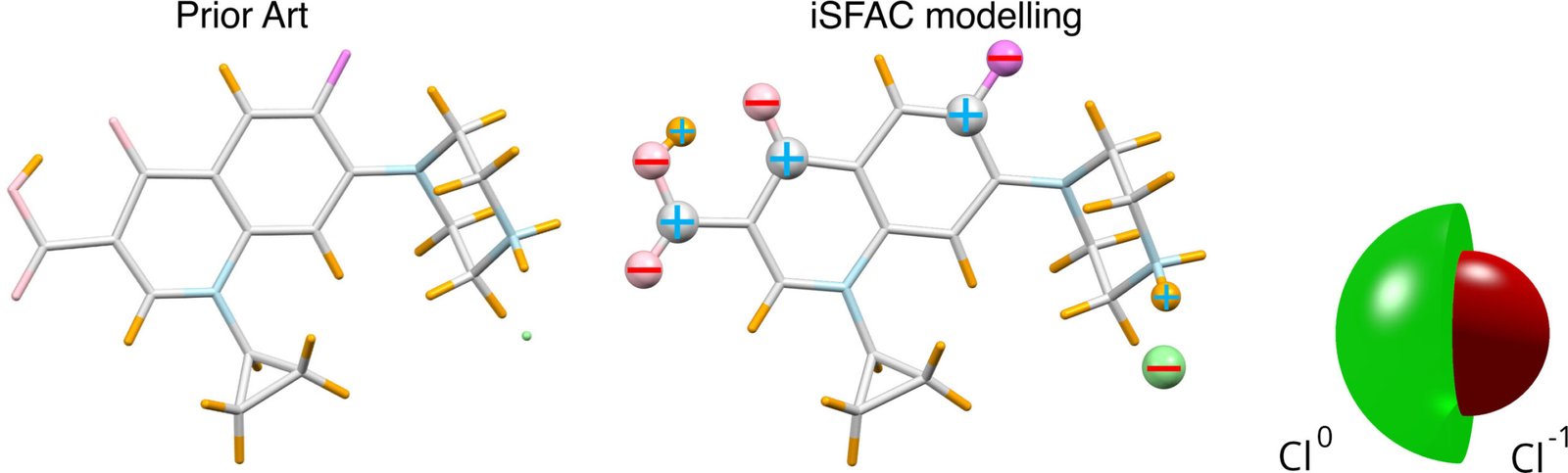
The team combined the diffraction data with a new analysis method called ionic scattering factor modeling (iSFAC). In this approach, each atom of a molecule is modeled simultaneously as a neutral and as a charged species. By comparing the model with the experimental data, the researchers were able to quantify the partial charge of each atom.

“Until now, partial charges were estimated using computational methods,” says Schröder. “Some of these fit atomic charges to reproduce the molecular electrostatic potential, resulting in so-called electrostatic potential-derived charges (ESP charges). Others partition the electron density itself among atoms.
“While widely used in molecular modeling, these methods can yield different values depending on the algorithm. Our new experimental technique now provides a means to assess and refine these theoretical models by offering a direct link.”
Broad applicability across molecular types
To demonstrate the broad applicability of their method, the researchers examined a diverse set of crystalline compounds—including the industrial catalyst ZSM-5, the amino acids tyrosine and histidine, tartaric acid from Austrian wine, and the widely used antibiotic Ciprofloxacin.
For Ciprofloxacin, which appears on the World Health Organization’s list of essential medicines and is commonly administered as a hydrochloride salt, the analysis showed that the chloride ion (Cl⁻), carries only about 40% of a full negative charge. This demonstrates how strongly a molecule’s environment can influence local charge distribution.
The Core Facility for Crystal Structure Analysis at the University of Vienna has played a key role in advancing electron crystallography in recent years. With this latest breakthrough, the technique moves beyond determining atomic positions to experimentally revealing electronic properties. The ability to measure partial charges opens new possibilities for designing pharmaceuticals with greater specificity and fewer side effects, as well as functional materials with precisely tuned properties.
More information:
Soheil Mahmoudi et al, Experimental determination of partial charges with electron diffraction, Nature (2025). DOI: 10.1038/s41586-025-09405-0
Provided by
University of Vienna
Citation:
Measuring how molecules communicate: New method quantifies partial charges (2025, August 21)
retrieved 21 August 2025
from https://phys.org/news/2025-08-molecules-communicate-method-quantifies-partial.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.